Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tsukimori, Kazuyuki
JNC TN9400 2000-086, 103 Pages, 2000/08
ln order to evaluate the integrity of the floor liner of "MONJU" at sodium leakage accident, nonlinear finite element analyses have been conducted considering the effect of thinning of the liner due to molten salt type corrosion. Modeling by shell elements is appropriate since liner is composed of thin plates, however, it is difficult to deal with the very local strain behaviors. 0n the other hand, modeling by solid elements makes the numerical calculation impractical. If we extract the small part of the liner which includes local discontinuities, it is possible to evaluate local strain behaviors practically by using the solid element analysis model of the part. To realize this approach, the method to generate all the boundary displacements of the part model from the shell element analysis result of total structure is needed. The aims of this study are to develop the method to deal with the incompatibility between shell and solid elements at part model boundary, and to build numerical analysis circumstances including this method to make the detailed nonlinear finite element analyses of the floor liner of "MONJU" possible. Summary of the results is shown below, (1)The problem of the incompatibility between shell and solid elements was solved by introducing weighting function at 'T' and 'L' type corners and the interpolation function of 4-node rectangular plate bending element at the connection between liner plate and frame. (2)Software system was developed by using 'FINAS' and verified. (3)This approach was applied to one of the cases of the floor liner analyses of "MONJU" at sodium leakage accident. The analysis result showed that three-dimensional local strain behavior could be evaluated directly. ln addition, it was confirmed that the result by shell element analysis was conservative in evaluation of strain compared with that by solid element analysis in this case.
 O
O -NaOH System)
-NaOH System)Yoshida, Eiichi; Aoto, Kazumi; Hirakawa, Yasushi;
JNC TN9400 2000-024, 42 Pages, 1999/10
For the purpose of improving the reliability of evaluation, the corrosion rate equation of the carbon steel SM400B (JIS G3106) in the high-temperature sodium compounds (NaOH-Na 0
0 system) was revised. ln this revision, the data acquired after 1997 was used. Based on the experimental results, the evaluation was made to be an approach to the following; (1)Metal loss of carbon steel in NaOH-Na
system) was revised. ln this revision, the data acquired after 1997 was used. Based on the experimental results, the evaluation was made to be an approach to the following; (1)Metal loss of carbon steel in NaOH-Na 0
0 system was evaluated as increases in exposure to the time, which is linear rate law. (2)There were no significant effects of the experiment atmosphere and mixing speed of the reagent on corrosion rate. (3)The concentration of Na
system was evaluated as increases in exposure to the time, which is linear rate law. (2)There were no significant effects of the experiment atmosphere and mixing speed of the reagent on corrosion rate. (3)The concentration of Na 0
0 in sodium compound is considered for the evaluation. The concentration under experiment is made to be the over concentration necessary for maintaining the dominant reaction between Fe and Na
in sodium compound is considered for the evaluation. The concentration under experiment is made to be the over concentration necessary for maintaining the dominant reaction between Fe and Na 20
20 . As a result of the evaluation, the additional data are 67 points. The data for the revision of the evaluation equation became the total of 105 points, when existing data of 38 points were added. The statistical evaluation of 105 points was carried out, and following recommended equation was obtained. C
. As a result of the evaluation, the additional data are 67 points. The data for the revision of the evaluation equation became the total of 105 points, when existing data of 38 points were added. The statistical evaluation of 105 points was carried out, and following recommended equation was obtained. C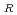 = C exp(-Q/RT) Where; C
= C exp(-Q/RT) Where; C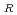 : Corrosion rate, mm/h C : Material constant Q : Apparent activation energy, cal/mol R : Gas constant, 1.986 cal/mol K T ; Absolute temperature, K Q = 9.61 kcal/mol C = 148.29 (average), 262.11 (99% UCL), 83.90 (99% LCL)
: Corrosion rate, mm/h C : Material constant Q : Apparent activation energy, cal/mol R : Gas constant, 1.986 cal/mol K T ; Absolute temperature, K Q = 9.61 kcal/mol C = 148.29 (average), 262.11 (99% UCL), 83.90 (99% LCL)